Response to the FDA’s CRL Request for Clarification of the
Astrocytoma Exposure Margins
1. INTRODUCTION
The following is a response to the FDA’s request in the CRL presented to Arena Pharmaceuticals to “provide additional data and information regarding the distribution of lorcaserin to the CNS in animals and human subjects that would clarify or provide a better estimate of astrocytoma exposure margins.” In the FDA briefing document section entitled: “Genotoxicity and Carcinogenicity Assessment for Lorcaserin” (6th paragraph; Brain Astrocytoma), Dr. Alavi (FDA’s nonclinical pharmacology/toxicity presenter) concludes:
“Given the absence of brain-to-plasma partitioning data in human subjects, the most conservative approach is to disregard estimated brain levels and rather calculate safety margins based on plasma drug levels, which is known for rats and humans. The safety margin to the non-tumorigenic dose would then be 5x, with brain tumors occurring at doses of lorcaserin 17x higher than clinical exposure.”
Dr. Alavi reached an incorrect conclusion when determining the safety margins for lorcaserin. His rejection of the brain-to-plasma partitioning based on the absence of human data is not supported by the scientific literature. Furthermore, his proposal to use plasma drug levels from rats and humans as the basis for the calculation is flawed.
First, it is true that there is only a limited amount of “brain-to-plasma partitioning data” available for humans, but it is incorrect to disregard estimated brain levels to calculate the safety margins for lorcaserin. As we will show, there is sufficient information in the Brain Drug Discovery literature to contradict Dr. Alavi’s statement. Second, it is not scientifically appropriate to “calculate safety margins based on plasma levels.” To do so in the way Dr. Alavi proposes would assume that plasma pharmacokinetic profiles are similar between rats and humans, when various studies have shown that there are in fact marked differences between the two species that affect comparisons of plasma concentrations of unbound drugs. Dr. Alavi’s proposed method to use trans-species comparisons of plasma drug concentrations also completely ignores the blood-brain barrier, which exhibits very low paracellular permeability and contains multiple bi-directional and/or uni-directional drug transporters. Thus, there are serious flaws underlying Dr. Alavi’s proposed method to determine the extent of drug penetration to the brain, and hence to determine the safety margins for lorcaserin with regard to brain astrocytomas. Although there are acknowledged limitations to estimating brain concentrations using the blood-to-plasma ratio, this method is a scientifically sound way to determine the margin of safety, based on what is available in the literature.
The intent of this paper is therefore to present the relevant literature regarding the reliability of available in vivo and in vitro tools, and the appropriateness of using animal models for determining drug concentrations in the brain. Providing this additional data and information should allow a better estimation of astrocytoma exposure to lorcaserin as requested by the FDA. Evidence-based studies will be the basis for recommending what methodology is the most reliable and the most feasible for the required purpose.
2. THE ISSUE: ASTROCYTOMA EXPOSURE SAFETY MARGINS
Here, we will review the facts about the Lorcaserin 2-year rat carcinogenicity study, in respect of astrocytoma incidence. The relevant data from both male and female rats are presented in Table of the FDA Briefing Document in the section entitled: “Genotoxicity and Carcinogenicity Assessment for Lorcaserin” (Brain Astrocytoma):
Table 11: Incidence of brain astrocytoma in 2 yr rat study,
main study groups (Control n=65, 10mg n=65, 30mg n=65, 100mg n=75)
Lorcaserin dose, mg/kg/day
Control 10 (5-fold) 30 (17-fold) 100 (55-fold)
Males 1 (1.5%) 0 4 (6.1%)NS 8 (10.7%)SS
Lorcaserin dose, mg/kg/day
Control 10 (7-fold) 30 (24-fold) 100 (82-fold)
Females 0 2 (3.0%) 0 1 (1.3%)
It is important to note that there were no drug related tumors in mice. In female rats, there was no statistically significant increase in astrocytoma incidence even though the Lorcaserin dose used in the high dose (HD) group was 82-fold that intended for humans. Despite concluding that the rat astrocytoma lineage was unresolved[1] and although a plausible tumorigenic mechanism was not identified, Dr.Alavi decided that human risk assessment for Lorcaserin would then be based on the difference in exposure between the tumorigenic dose in rats and the clinical dose.
Although Dr. Alavi states that it is more appropriate to compare the distribution of Lorcaserin in the CNS of rats and humans than to compare plasma drug levels, he then summarily rejects his own assertion and recommends that the safety margins should be calculated based on the plasma drug levels. Dr. Alavi agrees that it is reasonable to believe that brain partitioning in humans is better mimicked in monkeys than rats, but he then argues against the reliability of this method, based on his assumption that there are no human data to substantiate the belief. This point is important for the following reason: If it can be shown that brain partitioning in monkeys can be extrapolated to humans, then a reasonable margin of safety can be calculated from Tables 13 and 14 of the FDA Briefing Document in the section entitled: “Genotoxicity and Carcinogenicity Assessment for Lorcaserin” (Brain Astrocytoma):
Table 13: Brain partitioning of lorcaserin in rats and monkeys | |||
Species | Day of sampling | Brain to Plasma ratio (based on AUC) | |
Range | Average | ||
Rats1 | Day 1 | 13x - 35x | 26x |
Day 14 | 24x - 35x | 29x | |
Monkeys2 | Day 1 | 10x - 23x | 15x |
Day 14 | 8x - 12x | 10x | |
Humans | -- | Unknown | |
1Data source studies PDR-08218, 080097, & 08014. 2Data source study ARN-20080419.
Table 14: Estimated Safety Margins to Astrocytomas, based on estimated brain exposure to lorcaserin | ||||
Estimated Brain Exposure in Male Rats, ug*h/ml | Multiple to Estimated Brain Exposure in Humans at Clinical Dose (10mg bid) | |||
10mg/kg (No brain tumors) | 30mg/kg (brain tumors) | 10mg/kg (No brain tumors) | 30mg/kg (brain tumors) | |
115 - 168 | 405 - 591 | 11x - 17x | 40x - 59x | |
Estimated brain exposure in rats assumes 24x - 35x brain: plasma partitioning
Estimated brain exposure in humans assumes 10x brain: plasma partitioning.
It can be estimated from the above data that the safety margin for the non-tumorigenic Lorcaserin dose (i.e., where there are no brain tumors are present) would then be 17 x the clinical dose (10mg bid or 140 mg per day) with brain tumors occurring at doses of lorcaserin 59x higher than clinical exposure (1180 mg per day). Dr. Alavi rejects this calculation and recommends disregarding the estimated brain levels; his preference is instead to use the rat and human plasma levels to calculate the safety margin dose.
The literature reveals that brain partitioning in monkeys can, in fact, be used reliably. First, the blood-brain barrier is well conserved across vertebrate species. As an example “functional characteristics of these membranes are basically similar in all vertebrate classes” (Cserr HF 1984)[2]. Second, there is an excellent correlation between unbound drug brain fractions across species which is indicative of a high degree of conservation of the CNS environment (Summerfield, et al. 2008).Furthermore, various case studies demonstrate that brain tissue binding in homogenates used to determine the extent of brain drug penetration is comparable across species (Summerfield, et al. 2008). Shen presented evidence that no significant species differences occur in CNS disposition (Shen, Artru and Adkison 2004). In a study of CSF and plasma free concentrations for the determination of brain free concentration, the author concluded, “CSF penetration studies in animals can serve as predictive models in human drug development” (Liu, Smith, et al. 2006). With regard to cynomolgus[3] monkeys there is an extensive library of data that “genes expressed in cynomolgus monkey are highly conserved throughout primate evolution, and that virtually all had human homologs” (Osada, et al. 2001). The cynomolgus monkey is widely accepted to be closely related to humans and that, compared to rodents, they “exhibit greater similiarity to human physiology, neurobiology, and susceptibility to infectious and metabolic diseases (Gibbs, et al. 2007). The cynomolgus monkey is used extensively “in drug metabolism studies, because of its evolutionary closeness to humans” (Uno, et al. 2010) as evidenced by the similiarity of the respective genomes “with an average human-macaque sequence identity of ~93% (Gibbs, et al. 2007). These findings would be consistent with the FDA preclinical recommendations regarding the relevance of of drug toxicities in animals to humans:
“Another integral part of preclinical safety evaluation investigates the relevance of drug toxicities to humans. Toxicological findings often occur in some animal species, but not others. An animal species exhibiting similar drug profile (pharmacology and pharmacokinetics) to that of humans will be considered more relevant to humans. Toxicologicial findings in these relevant species will be given more consideration. When there is a lack of evidence of human relevancy among animal species, toxicological findings in the most sensitive species will then be given the most consideraton.”[4]
We also note that the FDA was involved in every step of the product development program,
including the early ones that are most relevant here. The animal studies were presented to the FDA, which was therefore informed about the details of, and rational for, the planned study.
Positron emission tomography (PET) has enabled drug distribution to be studied in the living brain, including human brains. The cynomolgus monkey is the primary animal model used in PET studies to determine CNS drug and radioligand tracer distributions for use in humans.[5] However, a complication of PET studies is the need to develop radio-labeled versions of drugs, which is not always possible on technical grounds. The assessment of brain concentrations of drugs in animal models using this imaging technique is further complicated by the possiblility that “labeled metabolites may complicate the interpretation of brain concentrations” (Eyal, Hsiao and Unadkat 2009). Although this field is promising it is still in it’s infancy. In the following sections, we will address the following questions: 1) How should the brain-to-plasam ratio (Kp) be used to assess brain penetration? 2) What are the most reliable and practical in vivo and in vitro methods, using animal models, to determine the extent of CNS drug penetration? 3) Is it reliable to use plasma drug levels to assess brain penetration and drug margin of safeyt?
3. HOW TO USE THE BRAIN-TO-PLASMA RATIO (Kp) TO ASSESS BRAIN PENETRATION
3.1 The Blood-Brain-Barrier and Central Nervous System (CNS) Physiology
The brain is separated, and protected, from the systemic circulation by two barriers – the blood-brain barrier (BBB) and the blood-cerebrospinal-fluid barrier (BCSFB). The BBB is a neurovascular unit (Neuwelt, et al. 2008) comprised of three cell types – endothelial cells, astrocytes, and pericytes. The capillary endothelium of the BBB is not a passive membrane, but rather it is an active organ that limits brain exposure to systemic substances through a network of tight junctions that are fundamental to how the BBB maintains homeostasis. All substances regardless of their chemical nature (e.g., ions, solutes, hormones) have to traverse this membrane, whose function is to “keep this traffic under control” (Liu, Chen and Smith 2008). Most of these subtances enter the brain by simple transcellar diffusion via a concentration gradient between the blood and brain (Di, Kerns and Carter 2008). It has been estimated that “98% of all small molecule drugs cross the BBB only negligibly, and miniscule amounts of large molecule drugs cross the BBB” (Neuwelt, et al. 2008). The BBB therefore limits the access of drugs to the brain interstitial fluid (ISF)[6]. The endothelial cells of the BBB differ from those in the rest of the body by lacking fenestrations and by having only sparse pinocytotic vesicular transport properties that, together minimize paracellular transport (Shen, Artru and Adkison 2004).
Protein Binding |
Metabolism |
Figure 1 Multiple mechanisms that affect brain penetration of molecules
Any description of the BBB would not be complete without describing the physiology and anatomy of the CSF[7]. The CSF is separated from the blood by the blood-cerebrospinal fluid barrier (BCSFB), which is formed by a continuous layer of polarized epithelial cells that line the choroid plexus. Of particular note is that the BCSFB is less quantitatively important than BBB transport in respect of most drugs that partition into the brain. Although there have been several reports on the use of CSF as a surrogate for the measurement of unbound drug concentrations in the brain (Liu, Smith, et al. 2006; Lin 2008; Liu, Chen and Smith 2008; Liu, Van Natta, et al. 2009;) it is not yet clear whether ISF and CSF concentrations can be accurately compared. Indeed, there are several reasons why ISF and CSF concentrations may differ (Shen, Artru, and Adkison 2004): 1) the ependymal lining the ventricles allows diffusional exchange from the CSF to the brain interstitium and the contribution of this exchange to the overall distribution of drugs is likely to be small; 2) the exchange between ISF and CSF is only diffusional (there are no facilitated transport mechanisms); 3) this exchange is counteracted by bulk flow of brain ISF to the CSF; 4) the diffusion distance from the CSF to most brain tissue is also considerably further than between the brain capillaries; 5) the concurrent transport of a drug across the BBB is likely to have a dominant effect on brain tissue concentrations after systemic administration because of the large area of the BBB and the short distance between brain capillaries. For these reasons, I will limit my discussion to the use of unbound and bound drug concentrations in brain homogenate as a surrogate for determining the same parameters in the living brain. It will be shown that this is the preferred and most reliable method to use for the present purpose. Of note is that the multiple mechanisms described above that affect the penetration of drugs into the brain are the main reasons why plasma-to-plasma comparisons should not be used to determine safety margins for CNS tumors, contrary to Dr. Alavi’s recommendations. In summary, P-gp substrates control the efflux and influx of compounds across the BBB; how a compound binds to proteins in the blood or plasma can significantly influence how much of it is present as a free entity in the blood; a compound that binds strongly to blood and plasma constituents could penetrate very poorly into the brain.
3.2 BBB Methodologies used in drug discovery
An excellent overview comparing the in vivo and in vitro models that have been developed to estimate brain penetration and exposure parameters can be found in Di, Li, Edward H Kerns, and Guy T Carter. "Strategies to assess blood-brain barrier penetration." Expert Opinion on Drug Discovery 3, no. 6 (2008): 677-87. Here I now summarize the methodologies used to determine the extent of brain drug penetration, highlighting their limitations and identifying the most current and reliable method.
3.2.1 Free Drug Hypothesis
This is the underlying principle that guides preclinical and clinical pharmacology. It is expressed in two parts. Part I states, “at steady state, the unbound drug concentration in plasma is the same as that in target tissues (e.g., brain, liver, muscle) when there are no transporters involved in the distribution process. Part II states, “the unbound drug at the site of action is the species that exerts pharmacological effects” (Di, Kerns and Carter 2008). As I shall show below, the unbound brain concentration is the most useful parameter for determining the extent of brain penetration. It is important to note that “the free drug hypothesis is supported by measurements, using the microdialysis technique, of the unbound drug concentration in the brain or other tissues and comparison with unbound concentration in plasma” (Lin 2008)[8]. Furthermore “it has been demonstrated that unbound brain drug concentrations most accurately predicted in vivo pharmacological activities for centrally active compounds” (Di, Kerns and Carter 2008). The most current thinking in the drug discovery field is to measure the concentration of unbound drug in the brain as the most reliable predictor of in vivo CNS drug penetration. As we shall see later, the total and unbound plasma and the total brain drug concentrations can be determined and be used as surrogates for estimating the brain penetration of a drug. The methodology to determine free drug concentrations involves the use of a brain homogenate in conjunction with equilibrium dialysis. The latter is an in vitro Kp dialysis method, which will be discussed in greater detail below.
3.2.2 In vivo methods for brain penetration
3.2.2.1 Brain perfusion assay. These assays are used to determine the rate of brain penetration and in situ brain perfusion. They are not commonly performed in drug discovery studies because they do not yield the concentration of unbound drug in the brain. Furthermore, the assay can be adversely influenced by the nonspecific binding of highly lipophilic drugs to brain tissue.
3.2.2.2 B/P ratio determination (Kp). This procedure will be discussed in more detail below.
3.2.2.3 Determination of CSF concentration. This method is not always useful as a surrogate way to determine the unbound drug concentration in the brain, because it is not always accurate. The limiting factor is the influence of efflux and influx transporters, which affect any estimate of brain drug concentration if equilibrium conditions are not established. In a recent study using CSF to assessing CNS exposure the author concluded, “CSF can only serve as a good surrogate for drugs that have good membrane permeability, but are not P-gp substrates…for drugs that are P-gp substrates or have poor membrane permeability, CSF cannot be used as a surrogate for assessing CNS exposure” (Lin 2008). In his study comparing unbound drug concentration in brain homogenate and CSF, Liu explains: “Because the drug transporters at the BBB and BCSFB are different, the CCSF (CSF concentration of drug) is not necessarily identical to the interstitial fluid” (Liu, Smith, et al. 2006). The discussion on CSF above is also relevant.
3.2.2.4 In vivo microdialysis. Microdialysis is a useful way to measure unbound drug concentrations in the brain, but it is not commonly used because of the following reasons: 1) it has a low throughput;[9] 2) it is resource consuming; 3) it is labor intensive; 4) non-specific binding of highly lipophilic compounds to the dialysis membrane can cause a low recovery of the drug.
3.2.3 In vitro assays for brain penetration
3.2.3.1 PAMPA-BBB. This method is included in the BBB permeability assay category; it is limited by a lack of BBB transporters because an artificial membrane is used.
3.2.3.2 MDR1-MDCKII and cell-based monolayer transport assays. Monolayer transport assays are useful tools for assessing the rate of brain penetration but do not measure the extent of brain uptake. They are designed to measure BBB permeability (they account for P-gp efflux), and so would not be useful for determining the rat astrocytoma exposure margins.
3.2.3.3 In vitro B-P (Kp) dialysis. This method will be discussed in detail below.
3.3 Brain-to-plasma ratio (Kp)
The brain-to-plasma ratio was the method used to estimate the brain concentration of Lorcarserin in rats and monkeys in the carcinogenicity studies. The traditional Kp ratio is based on a crude homogenization of brain tissue; it is expressed as the partition coefficient describing the total brain to total plasma concentration ratios.
The intent of the following discussion has its basis in Dr. Alavi’s statement to “disregard estimated brain levels and rather calculate safety margins based on plasma drug levels”[10] The literature suggests that although the Kp ratio has its limitations, it remains the method of choice in CNS drug discovery programs, even to this day. It is unclear on what basis Dr. Alavi decided to disregard the brain-to-plasma levels, because the Kp ratio “has been the in vivo gold standard in the industry and is routinely used to determine brain exposure for drug discovery programs” (Di, Kerns and Carter 2008). Humans have never been subjected to brain-to-plasma partitioning. All the results have been derived from animal experiments, and they are used in preclinical studies to evaluate potential CNS drugs. Using plasma levels exclusively to calculate safety margins is not supported in the literature. Not only did a Medline search fail to find any studies that used plasma-to-plasma concentration ratios to determine the extent of drug penetration into the CNS, not one study in this paper’s bibliography mentioned its use for this purpose.
In contrast, the following is a brief compiliation of quotes in the literature regarding the merits of the Kp method: 1) “Kp,brain is the most widely used in vivo parameter for assessing the extent of CNS distribution” (Kalvass, Maurer and Pollack 2007); 2) “Kp has been the most widely used parameter to evaluate and optimize brain penetration in drug delivery” (Liu, Chen and Smith 2008); 3) “Total brain concentrations (expressed as the Kp ratio) have historically been the most common method of measuring CNS exposure” (Hammarlund-Udenases, et al. 2008); 4) “A wide variety of models have been developed over the years to predict blood-brain-barrier (BBB) penetration, most of them focused on predictiong total concentrations of drug and then expressing this as a brain-blood (or plasma) ratio” (Jeffrey and Summerfield 2007); 5)”The brain-to-plasma concentration ratio (Kp) is the most commonly used parameter for measuring brain penetration in a drug discovery setting” (Becker and Liu 2006). Finally Reichel adds, “our past understanding of brain penetration was based heavily on measuring total brain levels…and is the most common method to study brain penetration in rodents” ( (Reichel 2009). In summary, pharmacokinetic (PK) support of CNS drug discovery relies heavily on the brain/plasma ratio in vivo, although there are some acknowledged limitations.
3.3.1 Kp limitations. Although Kp is a simple measure of brain partitioning and is the most common method used in CNS discovery programs, it neither provides a complete PK profile [i.e., maximal concentration (Cmax), half-life, and AUC] nor takes into account brain egress mechanisms (e.g., P-gp). In brain partitioning studies, it is commonly assumed that a drug with a large Kp ratio has extensive CNS penetration. Conversely, a Kp ratio less than 1 would mean that there is poor CNS penetration. However, Kp may be misleading because it does not take into account the “relative binding affinity of a substrate for proteins in plasma versus the proteins in the in the tissue in question” (Kalvass, Maurer and Pollack 2007). Alternatively, if the drug under evaluation has equal unbound plasma and brain concentration fractions then a Kp of 1 (known as unity) would be “consistent with the unrestricted distribution solely by passive processes” (Kalvass, Maurer and Pollack 2007). Therefore, the Kp ratio only provides a crude estimation of CNS drug levels because it does not take into account facilitated transport across the BBB or protein binding in plasma and brain. This has been confirmed in several case studies (Doran, et al. 2005; Liu, Chen and Smith 2008; Maurer, et al. 2005).
To summarize, factors that determine the extent of penetration of drugs into the brain are: 1) passive membrane permeability; 2) facilitated drug transport at the level of the BBB; 3) the degree of protein binding between plasma/blood and the brain; 4) metabolism; 5) the bulk flow of drug from the CSF (Figure 2).Thus, the Kp ratio is of limited value in the optimization of CNS drugs because it is a composite of all these factors. When using Kp in drug discovery to optimize brain penetration, the impact of plasma and brain protein binding must be understood. One must keep in mind that the goal in CNS drug discovery is to optimize the unbound drug concentration in the brain or the unbound brain-to-plasma ratio, and not the total (unbound/bound) brain-to-plasma ratio or the total brain concentration (Di, Kerns and Carter 2008). Despite this limitations, according to one expert, preliminary estimates of human Kp values derived from animal studies can be used as a suitable first approximation (Summerfield, et al. 2008).
In the Lorcaserin carcinogenicity study, Dr. Alavi dismissed brain-to-plasma partitioning and recommended using plasma concentrations from both rats and humans. However, this cannot be supported scientifically for two reasons: 1) there are no data to justify his assertion; 2) from the discussion regarding the BBB barrier it is clear that plasma drug levels cannot predict brain drug levels. In a study discussing methods to improve the prediction of human in vivo brain penetration the author compared the unbound fractions of a compound in plasma and brain across species and concluded, “For fu(plasma) [11] there is a more marked difference on comparing human and rat values (R2 = 0.49)”[12] On the other hand, “…a strong correlation ( R2 > 0.9) is noted between the unbound brain fractions when comparing any two of these species[13]” (Summerfield, et al. 2008). Thus there was a good correlation in the unbound drug brain fractions across species, whereas the unbound drug plasma fractions provided a poor correlation. Since the issue at hand is brain astrocytomas it is clear that Dr. Alavi’s recommendation is unjustified. The use of unbound drug plasma fractions and unbound drug brain fractions to determine the extent of CNS drug penetration will be the topic of the next section.
4. WHAT IS THE MOST RELIABLE AND PRACTICAL METHOD, USING ANIMAL MODELS, TO DETERMINE THE EXTENT OF CNS DRUG PENETRATION?
4.1 Introduction
The discussion on the properties of the BBB shows that research in this area is greatly needed to develop methodologies that can help those involved in CNS drug discovery programs have a better likelihood of success. A researcher aptly described this need, “Methods for investigating drug candidates in this field, from in vitro to in situ and in vivo, are under evaluation. However, confusion remains, and an intense debate is currently raging regarding how to interpret the results obtained and which methods to use to select candidates for central nervous system (CNS) action” (Hammarlund-Udenases, et al. 2008). All current thinking regarding the extent of CNS drug penetration is based on the principle of the free drug hypothesis: it is the unbound drug at the site of action that determines its efficacy. In addition, the concept of brain penetration benefits from considering a few key PK compartments within the brain - Fig 3.
Microdialysis has been considered to be the standard method in the measurement of ISF concentration (the brain extracellular fluid), which represents the unbound drug concentration in the brain. The main limitations of this method are not the result of unreliability but rather its high resource requirements and low throughput, together with the special surgical skills needed to set up the experiment (Liu, Van Natta, et al. 2009). As a result, researchers have been persuaded to explore alternative methods to determine the concentration of unbound drug in the brain. There are at least three methods that have been evaluated recently: brain homogenate, brain slice, and CSF.[14] A similar degree of accuracy was observed between the brain slice and brain homogenate methods (Becker and Liu 2006). This conclusion was also supported by the data reported by Friden et al. (2007). Because the brain slice method is more labor intensive and CSF has its limitations we conclude that of the three, the brain homogenate method is the most reliable and practical way to estimate the brain unbound concentration (Cu,brain).[15]
4.1.1 Brain homogenate as a surrogate for Cu,brain. A brain homogenate was introduced as a surrogate to estimate Cu,brain in a study on the influence of nonspecific plasma and brain binding on CNS exposure (Kalvass and Maurer 2002). In this approach, the Cu,brain is estimated from the unbound fraction in brain homogenate, as determined by using equilibrium dialysis or ultracentrifugation.[16]
Equilibrium dialysis can be performed in parallel with the estimation of the free fraction in plasma. This method can rapidly produce data on fu,brain (fraction unbound in brain) and fu, plasma (fraction unbound in plasma) for a large number of compounds and has a high throughput. These data allow the assessment of the extent of brain distribution in vivo. As the brain ISF contains only a very small amount of proteins, fu,brain can be used directly to estimate the unbound levels of a compound in brain ISF. Of note is that “there is very poor correlation between fu, plasma and fu,brain (Kalvass, Maurer and Pollack 2007). A possible reason for this discrepancy is the very different lipid and protein composition of plasma and brain, with plasma having twice as much protein, while the brain has 20-fold more lipids (Snyder, et al. 1975). The brain homogenate method, “which readily provides information on the free fraction of many compounds in brain tissue in vitro, has filled an important gap in our understanding of brain penetration and distribution” (Reichel 2009). This method allows routine access to drug concentrations in the brain.
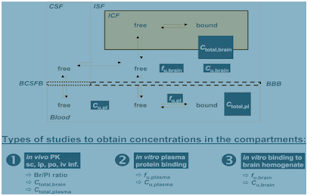
Figure 3 Principal pharmacokinetic compartments of the CNS, and the relation between bound and unbound concentrations in the compartments. The dark boxes illustrate parameters that can be measured in vitro and in vivo, and their relation to the concentration in these PK compartments. The three boxes at the bottom summarize the methods by which the parameters shown can be obtained: 1) in vivo determination of the total brain to total plasma ratio Kp, the total amount of drug in brain Atotal,brain , and total plasma concentrations Ctotal,plasma ; 2) and 3) equilibrium dialysis of blood plasma and brain homogenate giving the fraction unbound in plasma and brain, from which the unbound concentrations in
plasma Cu,plasma and brain Cu,brain from the in vivo study (1) can be derived (Reichel 2009)
4.1.2. In vitro assays: In relation to CNS penetration the in vitro methods are supplemented by two additional assays: 1) equilibrium dialysis of blood plasma; 2) equilibrium dialysis of brain homogenate (from the toxicological animal species) – Fig 4.
Figure 4 Schematic illustration – for our purposes only the equilibrium distribution on the right applies – the binding characteristics of the compound in blood plasma and brain tissue (homogenate) (Reichel 2009)
4.1.3 In vivo studies: The ‘classic’ total brain concentration vs. total concentration ratio (Kp ) should be determined. This has already been done in the Lorcaserin carcinogenicity study in the Rat Astrocytoma section. The resulting data will now be expressed in several ways, acknowledging the lessons learned from the ‘free drug hypothesis’ concept of brain penetration. First, the classic Kp is calculated. Using fu, plasma and fu,brain data from the in vitro study above, Kp is then transformed into the unbound Kp, uu (unbound brain to unbound plasma concentration ratio). The unbound brain concentration can be calculated. For the purpose of this study the unbound volume of distribution will not be commented on – Fig 5.
Figure 5 Schematic illustration of in vivo studies – for our purposes only study on the right as it relates to exposure in the brain (Reichel 2009).
4.1.4 Interpretation, Use, and Implication of Results. While the classic Kp value seems to be driven predominantly by nonspecific binding to brain lipids, its unbound counterpart Kp, uu is much more useful. It is the extent of the distribution equilibrium of a compound between the unbound fractions in brain and plasma. The Cu,brain (unbound concentration in brain, a surrogate for brain ISF levels) can be calculated for every time point where brain levels have been determined by correcting the total concentration in brain with fu,brain. (Reichel, 2009). In contrast to Kp values which are dose-static, Cu,brain values can be used to interpret dose-response results from efficacy studies and hence to establish dose/exposure/effect – Fig 6.
Figure 6 Interpretation and use of the brain PK parameters derived from both the first and second tier of in vitro assays and in vivo studies - see also the excellent reviews of Hammarlund-Udenaes et al. (2008) (Reichel 2009).
4.1.5 Summary and outlook: The following is a quote from Reichel (2009):
“The complex structure of the CNS makes brain penetration a very complex feature which cannot be rationalized on the basis of any single parameter. Although total brain concentrations are still the most common measure of CNS exposure, it is now emerging that they are more an indication of high nonspecific binding to brain tissue rather than being pharmacologically relevant concentrations. Neither total brain levels nor BBB permeability can be taken without considering the binding capacity of the brain tissue, when a link between exposure and efficacy is needed. The current paradigm of brain penetration is, therefore, changing towards a more compartmentalized view which allows a better rationalization of the distribution of compounds within the brain and makes use of brain compartments which are more relevant pharmacological effects.
Central to the emerging new paradigm is a clear differentiation between rate and extent of brain penetration, and between total and unbound drug levels as parameters for drug distribution within the brain. In attempting of a more holistic view, the concept is able to resolve a number of apparently contradicting observations, e.g., why CNS effects can be seen in the clinic, even though a CNS drug poorly permeates the BBB, is substrate of efflux pumps, has low total brain levels, is poorly bound to brain tissue, or has a very high plasma protein binding. Currently, there are also attempts to merge the presented concept of CNS penetration with the field of preclinical drug development and clinical PET to make extrapolations from animal to human more realistic."
5. IS IT RELIABLE TO USE PLASMA DRUG LEVELS TO ASSESS BRAIN PENETRATION AND DRUG MARGIN OF SAFETY?
An elegant study by Summerfield et. al. ( 2008) compared the unbound fraction of a series of compounds, 11 marketed CNS drugs and 10 PET tracers in brain homogenates from rats, Landrace pigs, and humans. There was a strong correlation (R2 > 0.9) [17] between the unbound brain fractions when any two of these species were compared, indicating that the method works well across the species.[18] This conclusion has been corroborated in other studies (Fichtl, Van Niecieki and Walter 1982; Haug 1987). However, there was a more marked difference when human and rat values for unbound plasma fractions are compared (R2=0.49), indicating that using drug plasma levels to predict penetration of these drugs into the CNS is unreliable. The different outcomes of the two methods is likely because of the “relative tissue affinities between plasma (or blood) and the brain” (Summerfield et al. 2008). The very poor correlation between unbound plasma fractions and unbound brain fractions has been confirmed by other investigators (Kalvass, Maurer and Pollack 2007).
The degree of tissue binding between plasma/blood and the brain can determine the availability of a drug in the brain. For example, a drug that binds strongly to blood and plasma constituents could penetrate very poorly into the brain. Other factors that govern the extent of brain penetration of drugs into the brain must also be considered: passive membrane permeability; facilitated drug transport at the level of the BBB; metabolism. Each of these factors can cause significant differences between the concentrations of a drug in the plasma and the brain. Another factor to consider is the different lipid and protein composition of plasma and brain; plasma has twice as much protein, the brain has 20-fold more lipids. These various biomolecules bind different drugs to different extents.
In summary, CNS penetration of drugs involves several factors that include plasma/tissue binding and the passage of tissue across the BBB. It has been demonstrated that “brain tissue binding appears comparable across species” and that “plasma binding varies more markedly from one species to another and is likely the better to reflect adaptation to environmental factors” (Summerfield et al. 2008). It is clear that recommending the comparison of plasma levels between rats and humans to calculate the margin of safety for lorcaserin with respect to astrocyte tumors is scientifically flawed.
6. CONCLUSION
The FDA requested additional data and information regarding the lorcaserin concentration in the CNS to better estimate astrocytoma margins. The request was prompted by the uncertainties about what is the most accurate method for calculating the margin of safety levels for lorcaserin, and about the appropriateness of using monkeys as the animal model for determining drug concentrations in the human brain. The FDA’s nonclinical pharmacology presenter disregarded monkey brain-to-plasma partitioning data in favor of comparing plasma drug levels between rats and humans, based on his rationalization that human data was not available.
Predictions of human pharmacokinetics have been traditionally been based on in vivo brain-to-plasma partitioning ratios (Kp) derived using animal models. This has long been the method of choice in CNS discovery programs, and it is still in use today. It is important to note that human brains have not been used in these models except in rare studies that have utilized cadaver brains or commercially available human brain specimens. The vast majority of CNS drugs that are used clinically today were developed using the Kp method, the in vivo gold standard in the drug discovery industry. Furthermore, a review of the literature confirms the wisdom of using this methodology. However, there are acknowledged limitations to the method, the most prominent being that it only provides the extent of total (bound and unbound) concentrations of drug in the brain. This caveat does not limit the method’s usefulness as a suitable first approximation for estimating the extent of drug penetration into the CNS, for the specific purpose of calculating the margin of safety levels for lorcaserin. It must be kept in mind that the goal in CNS drug discovery is to know the level of unbound concentrations that are available at the specific target sites of action. On the other hand, the Kp method is appropriate for determining drug concentration levels in toxicology studies. PET imaging is still an investigational method for such purposes.
The rationale for comparing plasma drug levels between rats and humans to determine astrocytoma exposure risks is flawed scientifically. Several case studies have shown that plasma protein binding varies markedly from one species to another, which means that the method is unreliable for determining CNS penetration and margin of safety values. Furthermore, using plasma levels excusively for this purpose is not supported in the scientific literature. Not only did a Medline search fail to find any studies that used inter-species plasma level comparisons to determine the extent of drug penetration into the CNS, not one study in the bibliography of a seminal review mentioned its use for this purpose. Furthermore, using only plasma levels ignores completely the role of the BBB as the gatekeeper to the CNS and the effect it can have on levels of compounds in the brain. The scientific literature strongly indicates that this method is inappropriate for the purpose proposed by the FDA’s examiner.
The appropriateness of using animal models for predicting drug concentrations in the human brain is not only supported by common practice in the CNS drug discovery industry, but also by the proven inter-species conservation of the BBB. The similar morphology and composition of the BBB across species has been widely documented. Several studies have confirmed that brain tissue binding is comparable across species in homogenates. Brain-to-plasma partitioning is therefore a valuable tool in drug discovery for providing early estimates of human brain concentrations; Kp values are estimated by combining human plasma (or blood) drug levels with animal brain tissue binding values. Furthermore, non-human primates are particularly suitable for this purpose as their close genetic relationship to humans is universally accepted; compared to rodents, their neurobiology is much more similar to humans. Non-human primates are used extensively in drug metabolism studies, in HIV research, and in vaccination studies. To disregard their use for our purposes is unfounded.
Finally, if the FDA is resistant to using the brain-to-partitioning ratio already determined for lorcaserin, despite the strong scientific rationale in its favor, then Arena Pharmaceuticals has the option of using a method involving brain homogenates and equilibrium dialysis for determining the unbound drug concentrations in the brain. This method has recently been introduced and is now widely accepted as the most reliable and practical way to estimate the unbound drug concentration in the CNS. It is a relatively simple procedure that has a high throughput, as described in detail above. This approach may even provide better margin of safety data because it does not measure the total (bound and unbound) drug in the CNS, unlike the simple Kp ratio. Thus, if the ratio between the brain unbound fraction and the plasma unbound fraction is lower (which is likely as it does not measure bound drug), then estimates of the drug levels in the brain would be reduced, increasing the corresponding estimates for lorcaserin’s margin of safety.
I would like to acknowledge Dr. John P. Moore for his assistance in editing this document.
Daniel P. Lopez, M.D., F.A.C.O.G.
Blue Ocean Research Group
References
Alavijeh, Mohammad S, Mansoor Chishty, M Zeeshan Qaiser, and Alan M Palmer. "Drug Metabolism and Pharmacokinetics, the Blood-Brain Barrier, and Central Nervous System Drug Discovery." NeuroRx 2, no. 4 (October 2005): 554-571.
Anan`eva, II, et al. "Glial tumors of the brain: current aspects of their classification and bases for genetic predisposition." Arkh Patol 69, no. 1 (Jan-Feb 2007): 54-60.
Becker, Stacey, and Xingrong Liu. "EVALUATION OF THE UTILITY OF BRAIN SLICE METHODS TO STUDY BRAIN PENETRATION." Drug Metabolism and Disposition 34 (February 2006): 855-861.
Cserr, HF, and M. Bundgaard. "Blood-brain interfaces in vertebrates: A comparative approach." American Journal of Physiology 246 (1984): R277-R288.
Di, Li, Edward H Kerns, and Guy T Carter. "Strategies to assess blood-brain barrier penetration." Expert Opinion on Drug Discovery 3, no. 6 (2008): 677-687.
Doran, A, et al. "The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: evaluation using the MDR1A/1B knockout mouse model." Drug Metabolism and Dispostion 33 (2005): 165-174.
Eyal, Sara, Peng Hsiao, and Jashvant D. Unadkat. "Drug interactions at the blood-brain barrier: fact or fantasy?" Pharmacology & Therapeutics 123, no. 1 (2009): 80-104.
Fichtl, B, A Van Niecieki, and K Walter. "Tissue binding versus plasma protein binding of drugs: General principles and pharmacokinetic consequences." Advances in Drug Research 20 (1982): 117-161.
Friden, M, A Gupta, M Anonsson, U Bredberg, and M Hammarlund-Udenaes. "In vitro methods for estimating unbound drug concnetrations in the brain interstitial and intracellular fluids." Metabolism and Disposition 35 (2007): 1711-1719.
Gibbs, Richard A., and et al. "Evolutionary and Biomedical Insights from the Rhesus Macaque." Science 316, no. 5822 (April 2007): 222-234.
Hammarlund-Udenases, M, Markus Friden, Stina Syvanen, and Anubha Gupta. "On The Rate and Extent of Drug Delivery to the Brain." Pharmaceutical Research 25, no. 8 (August 2008): 1737-1750.
Hassan, Moustapha, et al. "In vivo distribution of [11C]-busulfan in cynomolgus monkey and in he brain of a human patient." Cancer Chemotherapeutics and Pharmacology 30 (1992): 81-85.
Haug, H. "Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: A stereological investigation of man and his variability and a comparsion with some mammals (primates, whales, marsupials, insectivores, and one elephant)." American Journal of Anatomy 180, no. 2 (1987): 126-142.
Jeffrey, P, and G Summerfield. "Challenges for blood-brain barrier (BBB) screening." Xenobiotica 37, no. 10-11 (October-November 2007): 1135-1151.
Kalvass, Cory J, Tristan S Maurer, and Gary M Pollack. "Use of Plasma and Brain Unbound Fractions to Assess the Extent of Brain Distribution of 34 Drugs: Comparison of Unbound Concentration Ratios to In Vivo P-Glycoprotein Efflux Ratios." Drug Metabolism and Disposition 35 (2007): 660-666.
Kalvass, JC, and TS Maurer. "Influence of nonspecific brain and plasma binding on CNS exposure: implications for rational drug discovery." Biopharmaceutics & Drug Disposition 23 (2002): 327-338.
Lin, Jiunn H. "CSF as a Surrogate for Assessing CNS Exposure: An Industrial Perspective." Current Drug Metabolism, 2008 9 (2008): 46-59.
Liu, Xingrong, Cuiping Chen, and Bill J Smith. "Progress in Brain Penetration Evaluation in Drug Discovery and Development." The Journal of Pharmacology and Experimental Therapeutics 325 (2008): 349-356.
Liu, Xingrong, et al. "Evaluation of Cerebrospinal Fluid Concentration and Plasma Free Concentration As a Surrogate Measurement for Brain Free Concentration." Drug Metabolism and Disposition 34 (September 2006): 1443-1447.
Liu, Xingrong, et al. "Unbound Drug Concentration in Brain Homogenate and Cerebral Spinal Fluid at Steady State as a Surrogate for Unbound Concentration in Brain Interstitial Fluid." Drug Metabolism and Dispostion 37 (April 2009): 787-793.
Liu, Xingrong, Olga Vilenski, Joyce Kwan, Subbu Apparsundaram, and Robert Weikert. "Unbound Brain Concentration Determines Receptor Occupancy: A Correlation of Drug Concentration and Brain Serotonin and Dopamine Reuptake Transporter Occupancy for Eighteen Compounds in Rats." Drug Metabolism and Dispostion 37 (April 20 2009): 1548-1556.
Maurer, TS, DB Debartolo, DA Tess, and DO Scott. "Relationship between exposure and nonspecific binding of thirty-three central nervous system drugs in mice." Drug Metabolism and Dispositon 33 (2005): 175-181.
Neuwelt, Edward, et al. "Strategies to advance translational research into brain barriers." Lancet Neurology 7 (2008): 84-96.
Osada, N, et al. "Assignment of 118 novel cDNAs of cynomolgus monkey brain to human chromosomes." Gene 275, no. 1 (September 2001): 31-37.
Reichel, Andreas. "Addressing Central Nervous System (CNS) Penetration in Drug Discovery: Basics and Implications of the Evolving New Concept." Chemistry & Biodiversity 6 (2009): 2030-2049.
Shen, Danny D, Alan A Artru, and Kimberly K Adkison. "Principles and applicablility of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics." Advanced Drug Delivery Reviews 56 (2004): 1825-1857.
Snyder, WS, MJ Cook, ES Nasser, GP Karhausen, I Howellls, and H Tipton. Report of the Task Force on Reference Man. Oxford: Pergamon Press, 1975.
Summerfield, S.G, et al. "Toward an improved prediction of human in vivo brain penetration." Xenobiotica 38, no. 12 (2008): 1518-1535.
Uno, Yasuhiro, Shotaro Uehara, Sakae Kohara, Norie Murayama, and Hiroshi Yamazaki. "Cynomolgus Monkey CYP2D44 Newly Identified in Liver, Metabolizes Bufuralol, and Dextromethorphan." Drug Metabolism & Disposition 38 (September 2010): 1486-1492.
[1] It is interesting to note that although malignant astrocytic gliomas account for 50% of all primary brain tumors in humans the cells of origin are mostly unknown (Anan`eva, et al. 2007)
[2] This is a meticulously detailed article, a mine of information that is supported by a range of scholarly literature surveyed and assessed that is encyclopedic.
[3] This is the species of monkey used to determine brain-to-plasma brain partitioning ratios. It is an Old World macaque monkey.
[4] Downloaded from the FDA website at http://www.fda.gov/ohrms/dockets/ac/03/briefing/3976B1_02_F-FDA-Tab%205.pdf
[5] There are numerous reports of these PET studies, but for the present purpose a Medline search is appropriate.
[6] The interstitial fluid (ISF) is the brain extracellular fluid that is in direct continuity with brain tissue is separated from the cerebrospinal fluid via the brain-cerebrospinal-fluid barrier (BCFB).
[7] This discussion will be based on excellent review in Hammarlund-Udenases, M, Markus Friden, Stina Syvanen, and Anubha Gupta. "On The Rate and Extent of Drug Delivery to the Brain." Pharmaceutical Research 25, no. 8 (August 2008): 1737-50.
[8] Li Di mentions that this is a brilliant review on BBB concepts and applications of CSF as a surrogate for unbound brain drug concentration in drug discovery.
[9] Throughput refers to the number of tests that can be performed in a given time.
[10] From the FDA briefing document section entitled: “Genotoxicity and Carcinogenicity Assessment for Lorcaserin” (6th paragraph; Brain Astrocytoma).
[11] fu(plasma) describes the unbound fraction of a compound in plasma.
[12] A statistical measure of how well a regression line approximates real data points; an r-squared of 1.0 (100%) indicates a perfect fit. Here it is used to determine correlation of drug plasma and brain concentrations among various species of animal models.. An R2 of one denotes a perfect correlation.
[13] The comparison was between rats, Landrace pigs, and human (human brain samples were obtained from Biomedical Solutions).
[14] The limitations of determining the unbound concentration in the CSF were discussed earlier.
[15] The discussion in this section will concentrate primarily on the brain homogenate method. It is just as reliable as the other two and it can be much more easily implemented in the drug discovery setting. I will also summarize the most current articles on this subject in the listed references (Becker and Liu 2006; Friden, et al. 2007; Liu, Van Natta, et al., 2008; Summerfield, et al. 2008; Hammarlund-Udenases, et al. 2008; Reichel 2009; Jeffrey and Summerfield, 2009; Liu, Vilenski, et al. 2009).
[16] The in vitro unbound fraction in brain homogenate and plasma for each compound was determined using a 48-well Rapid Equilibrium Dialysis device (Linden Bioscience, Woburn, MA). Brain tissue was homogenized in 2 volumes (w/v) of 0.9% saline. Brain homogenate or plasma was spiked with a compound for a concentration of 1000 ng/ml. Two-hundred
microliters of the matrix was added to the donor side of a dialysis chamber. The receiver side contained 350 µl of Sorenson’s buffer. The dialysis apparatus was maintained on a shaking device at 37°C for 4 h. The drug concentrations
were determined as described in (Liu, Van Natta, et al. 2009).
[17] A statistical measure of how well a regression line approximates real data points; an R2 value of 1.0 (100%) indicates a perfect correlation. Here it is used to determine the correlation between drug plasma and brain concentrations among various species of animal models.
[18] Of note, is that this is further evidence supporting the use of cynomolgus monkeys as a predictive animal model for human brain drug concentrations.